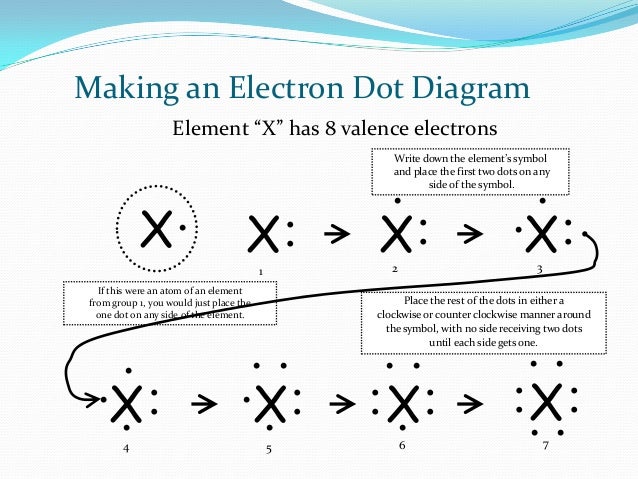
The number of dots you have around the Lewis electron-dot symbols of elements represents the actual number of valence electrons for the element.
Elements belonging to Group VIIIA are called the noble gases. These elements have 8 valence electrons and are said to have a complete octet of electrons. This is the equivalent of a filled energy level, or a closed shell. This special arrangement of EIGHT electrons makes the noble gases relatively unreactive. Although Helium is the only noble gas with two valence electrons, you will recall that the first energy level can only accommodate two electrons.
A thorough understanding of the Rule of Eight or the Octet Rule is essential for further study involving chemical bonding of atoms and the formation of compounds. It turns out that in the formation of molecules from atoms, most atoms attempt to achieve this configuration of 8 valence electrons around each atom. In order to achieve this, atoms tend to lose or gain electrons to ions in order to achieve an octet of electrons.
.For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations.For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions.For atoms with 4 valence electrons, it can go either way.For atoms with 8 valence electrons, there is no change.
Ion formation
| Group | Number of valence electrons | Gain or lose electrons | Charge of ions | Examples |
|---|---|---|---|---|
| IA | 1 | lose 1 electron | +1 | H+, Li+, Na+, K+ |
| IIA | 2 | lose 2 electrons | +2 | Mg+2, Ca+2, Sr+2 |
| IIIA | 3 | lose 3 electrons | +3 | Al+3, Ga+3 |
| IVA | 4 | lose 4 electrons gain 4 electrons | +4 -4 | Sn+4, Pb+4 C-4 |
| VA | 5 | gain 3 electrons | -3 | N-3, P-3 |
| VIA | 6 | gain 2 electrons | -2 | O-2, S-2 |
| VIIA | 7 | gain 1 electron | -1 | F-, Cl- |
| VIIIA | 8 | Complete octet makes the noble gases unreactive | ||
- Jun 20, 2020 The valence shell of aluminum has three electrons, and per the octet rule, these three electrons are lost resulting in just 10 electrons and 13 protons.
- There are 3 valence electrons in the atom of aluminum. Number of valence electrons depends on number of electrons in uncharged atom (= atomic number), not on mass. Chat Now Send Inquiry How many valence electrons does aluminum have?.
Number of bonds
The location of an element in the periodic table allows us to predict the number of covalent bonds that it can form. When an element forms a bond with another element, a line is drawn to represent the sharing of a pair of electrons between the two elements.

- Hydrogen belonging to Group IA has one valence electron, shares this valence electron with another atom to form one covalent bond.
| Electron - Dot Symbol | Covalent Bonding | Number of Covalent Bond |
|---|---|---|
| 1 |
- Boron belonging to Group IIIA has three valence electrons, shares its valence electrons with other atoms to form three covalent bonds.
| Electron - Dot Symbol | Covalent Bonding | Number of Covalent Bond |
|---|---|---|
| 3 |
- Carbon and Silicon belonging to Group IVA have four valence electrons, shares their valence electrons with other atoms to form four covalent bonds.
| Electron - Dot Symbol | Covalent Bonding | Number of Covalent Bond |
|---|---|---|
| 4 | ||
| 4 |

- Nitrogen and Phosphorus belonging to Group VA has five valence electrons, shares three of its five valence electrons with other atoms to form three covalent bonds. The other two valence electrons form one nonbonding electron pair.
| Electron - Dot Symbol | Covalent Bonding | Number of Covalent Bond |
|---|---|---|
| 3 | ||
| 3 |
- Oxygen and Sulfur belonging to Group VIA has six valence electrons. These elements need two valence electrons to complete an octet of electrons. They share two of the six valence electrons with other atoms to form two covalent bonds. The other four valence electrons form two nonbonding electron pair.
How Many Valence Electrons In Aluminum
| Electron - Dot Symbol | Covalent Bonding | Number of Covalent Bond |
|---|---|---|
| 2 | ||
| 2 |
Number Of Valence Electrons In Aluminum Ion
- Fluorine, Chlorine, Bromine and Iodine belonging to Group VIIA has seven valence electrons. These elements need one valence electron to complete an octet of electrons. They share one of the seven valence electrons with other atoms to form one covalent bond. The other six valence electrons form three nonbonding electron pair.
| Electron - Dot Symbol | Covalent Bonding | Number of Covalent Bond |
|---|---|---|
| 1 | ||
| 1 | ||
| 1 | ||
| 1 |
Exceptions to the Octet Rule
There are exceptions to the Octet rule for atoms below period two.
- Boron and aluminum compounds commonly have six electrons around the metal center (eg AlH3, BH3). Compounds with less than an octet around each atom are called electron deficient. Part of this is due to their low electronegativity properties.
- Phosphorus and Sulfur have a tendency to exceed their octets (eg PF5, SF6). Phosphorus and sulfur expand their octets to accommodate more than eight electrons because they have empty 3d orbitals.
The Octet Rule is a simplistic view on the concept of bonding. Molecular Orbital Theory provides a better description of the complexity of molecular bonding.
Content suitability
BCIT courses:CHEM 0011
